Systems for Intermediate Temperature Storage for Fracture Reduction and Avoidance
Cryonics, 3rd Quarter 2011
See also the Chronology of Developments Related to Fracturing and Intermediate Temperature Storage at the end of this document.
By Brian Wowk, PhD
Introduction
Cryopreservation by vitrification partially replaces water inside cells and tissue with chemicals called cryoprotectants that prevent ice formation. At high enough concentrations, cryoprotectants can prevent freezing. Instead of freezing, the mixture of water and cryoprotectants becomes more and more viscous like syrup during cooling. At a temperature near -120°C the viscous solution solidifies, an event called the “glass transition.” This solidification without freezing is the physical basis of cryopreservation by vitrification.
Liquid nitrogen provides an inexpensive, stable, and highly reliable storage environment for cryopreserved tissue at a temperature of -196°C. Unfortunately the process of cooling to this very cold temperature tends to cause cryopreserved tissues to fracture. Such fractures probably do not compromise the neuroanatomical information preservation goals of cryonics as long as tissue remains cold and solid. However fractures prevent future recovery of cryopreserved tissue by any simple means. They are also alarming by contemporary biomedical standards.
Fracturing can be reduced, and sometimes avoided, by cooling through the glass transition temperature slowly and stopping cooling at temperatures warmer than liquid nitrogen. Much progress has been made within the past decade at developing systems able to safely store tissue at temperatures warmer than liquid nitrogen. Such systems have come to be called “intermediate temperature storage” (ITS) systems because they store at temperatures intermediate between liquid nitrogen and the glass transition temperature. ITS technologies are more complex and expensive than simple immersion in liquid nitrogen. Although ITS technologies for cooling and storing tissue in relative safety at adjustable temperatures now exist, the basic science knowledge of how to control temperature to avoid fracturing in tissues as large as a whole human body still does not exist. Only fracture reduction is presently possible.
Physical Causes of Fracturing
The vibration of molecules gives rise to a characteristic volume, or density, of a liquid or solid material at a given temperature. As temperature decreases, the volume of an object slightly decreases. This is called thermal contraction. Figure 1 shows thermal contraction of a cryoprotectant solution cooled in a test tube. Warmer solution in the center of the test tube continues cooling and contracting after solution near the walls has solidified, creating a dimple.

Fig. 1. Differential thermal contraction of vitrification solution cooled in a test tube causes a dimple to form. The warmer inside of the solution continues contracting after the colder outside has solidified and begun cooling at a slower rate.

Fig. 2. A two liter volume of solidified M22 vitrification solution shown (a) just below the glass transition temperature and (b) after further cooling. The solidified solution fractured during further cooling below the glass transition temperature.
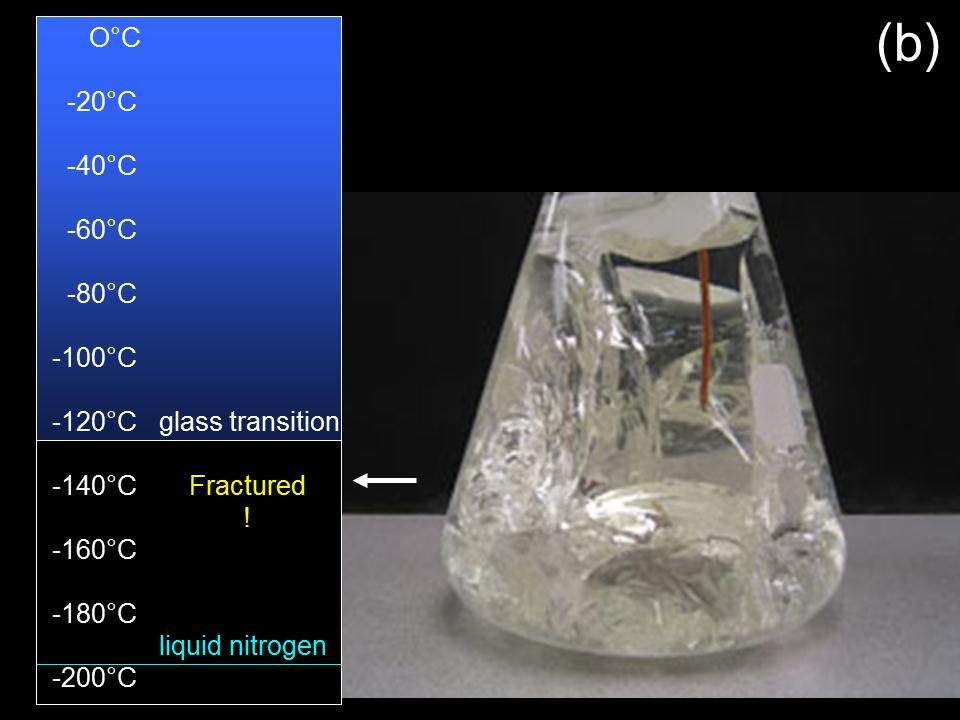
Thermal contraction can cause cryoprotectant glasses (solidified vitrification solution) to fracture by several different mechanisms (1). Figure 2 shows a cryoprotectant solution in a borosilicate glass flask fracturing after vitrification and cooling. This fracturing occurred because the cryoprotectant solution adhered to the glass wall when it solidified. Cryoprotectant glasses have thermal expansion coefficients ten times greater than the flask container walls. Therefore cryoprotectant glasses will shrink ten times as much during cooling as glass containers that hold them. The cryoprotectant glass in Fig. 2 broke due to accumulated stress as it tried to retract away from the flask wall during cooling. Vitrified cryoprotectant solutions or tissues are less likely to fracture if held in containers made of hydrophobic materials that solutions don’t adhere to, such as polyethylene plastic.
Cryoprotectant glasses or vitrified tissue can also fracture due to internal stress during temperature change regardless of container material. If different parts of tissue have different thermal expansion properties, the different parts will seek to contract by different amounts during cooling, causing stress that can result in fractures.
Even completely homogeneous tissue or pure cryoprotectant solutions can fracture during cooling. If different parts of a material cool at different rates, the rates of thermal volume contraction will be different. For example, near the end of cooling, the outside of an object may only contract at 0.1% per minute as its temperature nears that of the surroundings. However the inside of the object may be trying to contract at 0.2% per minute because it is warmer and still cooling faster . The core of the object therefore tends to pull away from the periphery, causing mechanical stress, which causes fracturing if the mechanical strength of the solid is exceeded. This phenomenon is illustrated schematically in Fig. 3.

Fig. 3. As cooling slows at the cold exterior of a vitrified object, the warmer interior will cool faster until a uniform temperature is reached throughout the object. Faster interior cooling causes faster thermal contraction of the interior, causing the interior to pull away from the exterior along the dotted line. These forces can cause the vitrified object to fracture.
In practice, it’s difficult to cool volumes of more than a few milliliters of vitrification solution to the temperature of liquid nitrogen without fracturing. This is because the thermomechanical properties of cryoprotectant glasses (thermal expansion coefficient 40 x 10-6 per °C, fracture strain 0.3%, fracture stress 3 MPa (5, 6)) make them much weaker than other glasses we are accustomed to. For example, window glass has a fracture stress of approximately 100 MPa due to its strong covalent chemical bonds.
Fibrous material present in a vitrification solution will increase the vitrified solution’s fracture strength, and reduce the likelihood and extent of fracturing. Tissue itself is fibrous, so tissues and organs generally do not fracture as easily or extensively as bare cryoprotectant solutions like the solution in Fig. 2(b). However organs are still capable of fracturing during cooling.
Prevelance of Fracturing in Cryonics
In late 1983 Alcor performed postmortem examinations of three whole body cryonics patients who had been transferred from another cryonics facility for conversion to neuropreservation and continued storage at Alcor. In every patient, several full thickness fractures of the skin were observed as well as multiple fractures of most internal organs. The spinal cord of one patient was cleanly fractured every 6 cm over a 20 cm length examined. These patients were frozen with low concentrations of cryoprotectant rather than vitrified, so fracturing is a phenomenon that can occur in either frozen or vitrified tissue during cooling to liquid nitrogen temperature. It is not unique to vitrification. These findings were documented in a report in the September 1984 issue of Cryonics magazine (7). There was further discussion of these findings on page 28 of the 1st Quarter 1995 issue of Cryonics (8).
In 1994 Alcor performed a postmortem examination of the brain of Alcor patient A-1242 who had been ordered removed from cryopreservation after a court overturned the 1990 cryopreservation arrangements made by her husband. It was discovered that the brain had fractured into five major pieces. Details were reported on page 29 of the 1st Quarter 1995 issue of Cryonics (8).

Fig. 4. Acoustic events believed to be fractures detected in the brain of Alcor patient A-2063 during cooling after perfusion with B2C vitrification solution. Such events are detected in all patients during cooling between the glass transition temperature (-123°C) and liquid nitrogen (-196°C).
In 1997 Alcor brought into regular use an acoustic fracture detection system called the “crackphone.” The crackphone is a custom designed system that performs digital data processing of sound signals recorded by microphones placed in contact with the brain during deep cooling of cryonics patients. It detects and records acoustic events believed to correlate with fracturing.
Acoustic events consistent with fracturing were found to be universal during cooling through the cryogenic temperature range. They occurred whether patients were frozen or vitrified. If cryoprotection is good, they typically begin below the glass transition temperature (-123°C for M22 vitrification solution). If cryoprotective perfusion does not go well, then fracturing events begin at temperatures as warm as -90°C. Higher fracturing temperatures are believed to occur when tissue freezes instead of vitrifies because freezing increases the glass transition temperature of solution between ice crystals. The temperature at which fractures begin is therefore believed to be a surrogate measure of goodness of cryoprotection, with lower temperatures being better.
The crackphone is believed to be highly sensitive to fractures, but its specificity is not clear. Cracking sounds can often be heard during cooling of vitification solutions or vitrified tissue with no fractures later being found (unpublished observations of the author). So it is not clear whether every acoustic event detected during cooling is necessarily a fracture. Studies correlating acoustic events with physical fracturing have not been done. Still, it is believed that the brain and other major organs of every cryonics patient cooled to the temperature of liquid nitrogen to date have some fractures.
Significance of Fracturing
Fracturing does not cause tissue to break into widely separated pieces at the time of fracture. As shown in Fig. 5, fractures are not macroscopically obvious at cryogenic temperature. The actual physical displacements associated with fracturing are small, and are believed to remain small as long as tissue remains solid. Future repair strategies for fracturing are therefore anticipated to begin at low temperature with the tissue still in the solid state (2, 3).

Fig. 5. Vitrified brain of Alcor patient A-2077 under liquid nitrogen. This brain is almost certainly fractured, yet it remains an integrated whole. Movements between fracture planes appear to remain microscopic provided that tissue stays cold and solid.
Fractures in bare cryoprotectant solutions such as those in Fig. 2 are observed to be optically smooth. In other words, the fracture surfaces are smooth on a scale smaller than a wavelength of light, which is less than one millionth of a meter. Although the fracture faces of vitrified tissue have not been specifically studied, it is assumed that they are also relatively smooth. When frozen tissue is fractured for microscopy in a procedure called “freeze fracture,” the resulting faces are smooth enough for electron micrographic study of cell membranes. From an information theoretic standpoint, it seems likely that fracturing does not cause loss of neural connectivity information provided that tissue remains vitrified, and provided that some future means exists to match and restore structure across fracture faces. However further study is needed.
Unfortunately fracturing excludes any future repair strategy that might begin by simple warming and reperfusion. It’s therefore a barrier to the development of reversible suspended animation of large organs or humans no matter how good cryoprotectant technology becomes. Fracturing underscores that cryonics as currently practiced is an information archiving technology that will require very arcane technology to reverse. It is not anything close to suspended animation.
Reduction and Elimination of Fracturing
To prevent fracturing, stresses such as those shown in Fig. 3 need to be minimized. This can be achieved by slowing cooling as the glass transition is approached so that the temperature is as uniform as possible within tissue during descent through the glass transition. Holding for a period of time near or just below the glass transition to allow stress relaxation before further cooling is especially helpful. Figure 6 shows a cooling protocol that permitted storage of a rabbit kidney under liquid nitrogen with no evidence of fracturing during later transplantation (4). Faster cooling has been observed to result in fracturing.

Fig. 6. Slow cooling and warming protocol followed for a vitrified rabbit kidney that was successfully stored under liquid nitrogen for two weeks prior to transplantation without fractures. (Data courtesy 21st Century Medicine, Inc.)
Another strategy has allowed even bare vitrification solutions, which are highly susceptible to fracturing, to reach liquid nitrogen temperature without fracturing. That strategy is to cool slightly below the glass transition temperature, then rewarm above it, and then finally resume cooling as shown schematically in Fig. 7. This allows interior temperatures to catch up to the cooler exterior temperature so that the whole object passes through the glass transition at a more uniform temperature and cooling rate. This avoids “locking in” stresses that would otherwise result from non-uniform passage through the glass transition.
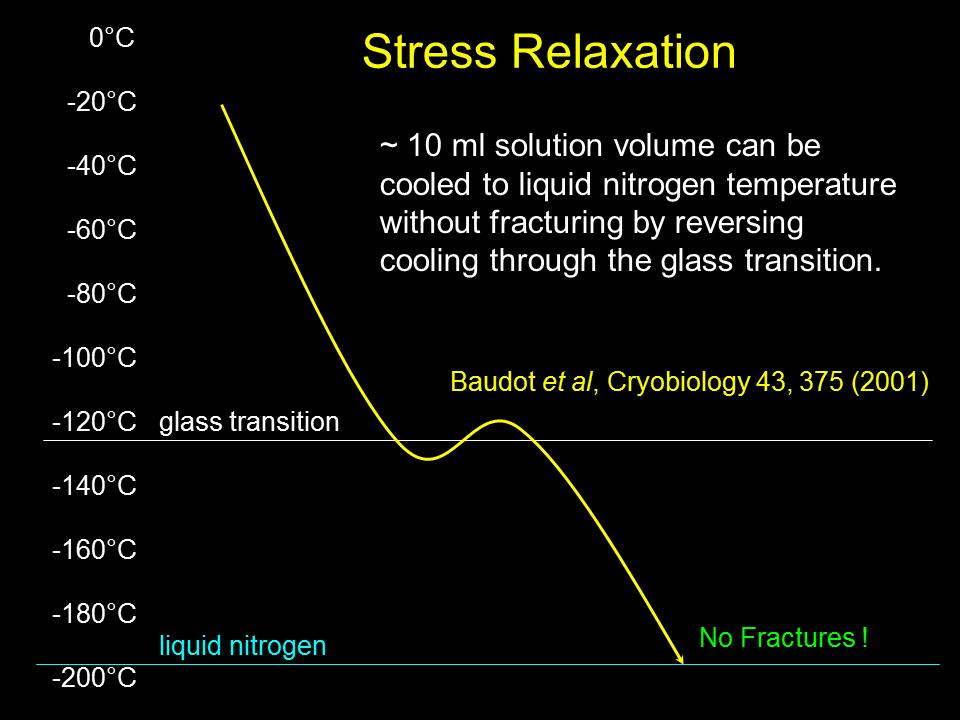
Fig. 7. Warming above the glass transition temperature after descending slightly below it can reduce temperature gradients and associated stress, allowing 10 mL solution volumes to reach liquid nitrogen temperature without fracturing.
Molten silicate glass (window glass) that is cooled too quickly will also fracture for the same reasons that cryoprotectant glasses do. To prevent this, silicate glasses are held during manufacturing for a period of time near their glass transition temperature to reduce stress. This process is called annealing. After annealing and slow cooling to a lower temperature, called the strain temperature, silicate glass can be quickly cooled to room temperature without fracturing. A similar annealing process allowing cooling of large volumes of cryoprotectant glasses to liquid nitrogen temperature without fracturing is theoretically possible. Unfortunately, due to the physical weakness of cryoprotectant glasses compared to silicate glass, very long annealing times may be necessary.
Whether for long periods of annealing or permanent storage, systems for storing cryopreserved tissue at temperatures between the glass transition temperature and liquid nitrogen temperature are necessary if fracturing is to be avoided. In cryonics, such systems have come to be called Intermediate Temperature Storage (ITS) systems.
Progress in Development of Intermediate Temperature Storage (ITS) Systems
For decades mechanical laboratory freezers have been available that are capable of maintaining temperatures as low as -140ºC. They’ve been sold under names such as Queue and CryoStar. In the year 2000 a 10 cubic foot CryoStar freezer was acquired for testing by Alcor for possible use storing neuropatients. It included a liquid nitrogen backup system able to maintain temperature in the event of a power failure, and was also filled with dry ice as thermal ballast. Alcor used it for two patients between 2002 and 2006 before advancing to newer liquid nitrogen ITS systems. The newer systems had much lower power consumption, no temperature cycling, and other advantages described below.
“Vapor phase” storage systems that store at temperatures warmer than liquid nitrogen in the vapor space above liquid nitrogen have long been available. However as shown in Fig. 8(a), they suffer from large uncontrolled temperature differences in the vapor space. They are used in cryobiology not because of their warmer temperature, but because they prevent transfer of pathogens between samples stored under a common pool of liquid nitrogen.
Figures 8(b) and 8(c) show that if an insulated container with a conductive inner liner is placed above liquid nitrogen, the non-uniform temperature outside the container becomes converted into a more uniform temperature inside the container. The addition of a small thermostat-controlled electric heater inside the container as shown in Fig. 8(d) allows the uniform interior temperature to be adjustable. 21st Century Medicine, Inc., obtained US Patent 7,278,278 for this and related types of intermediate temperature storage systems in 2007.

Fig. 8. (a) The temperature in the vapor space above liquid nitrogen. (b) More uniform temperature inside an insulated storage container with a thermally-conductive inner liner. (c) Calculated heat flows inside container. (d) Adjustment of container temperature with electrical heat.

Fig. 9. Prototype neuropod suitable for maintaining single neuropatients at a stable and adjustable intermediate storage temperature, (a) showing neurocan inside, (b) with top insulation in place, (c) and (d) inside a small dewar with 8 liters of liquid nitrogen at the bottom able to maintain -140°C inside the neuropod for 90 hours between refills. Longer times between refills are possible with larger dewars. The blue cable connects to a small temperature controller that supplies electrical heat to the inside of the neuropod to maintain the desired interior temperature. The neuropod requires 0.15 watts heating for each °C temperature difference between the interior and mean exterior temperature.
In 2003, Alcor acquired the prototype “neuropod” storage device shown in Fig. 9 for testing. Using the principles explained in Fig. 8, the neuropod was designed to hold a single neuropatient at an adjustable, uniform, and stable intermediate temperature. The neuropod itself could be placed into any uncontrolled cryogenic environment, such as the vapor space above liquid nitrogen in a conventional storage dewar. An electric heater supplied by a temperature controller added small amounts of heat as necessary to maintain the desired temperature inside the neuropod. The heat automatically adjusts to maintain a stable internal temperature even when the outside temperature fluctuates, such as during dewar refilling (temperature drop) or transfer through ambient air between dewars (temperature rise). The power requirements of the controller are so low (<10 watts) that they can easily be met by small battery backup systems.
The advantages of this type of ITS system are numerous.
- No moving parts
- Low power requirements
- Individual temperature control
- Power failure results in cooling rather than warming
- Temperature stability in presence of external instability or non-uniformityStorage flexibility (containers will function in any cryogenic environment that is on average colder than the target interior temperature)

Fig. 10. Collections of neuropods could be stored in the vapor space of conventional liquid nitrogen dewars. The temperature inside each one could be individually controlled to manage complex cooling plans for annealing protocols lasting years if necessary.
In 2004 Alcor acquired and began testing another neuropod that was designed to be “patient rated.” It incorporated dual redundant temperature controllers and heaters, and other safety features.
The advantage of individual storage pod temperature control is a disadvantage in terms of complexity and cost. An alternative approach is to construct an intermediate temperature storage system that maintains a large common volume at the same temperature. In 2003 21st Century Medicine, Inc., developed and constructed an ITS storage dewar for cryobiology applications capable of maintaining adjustable and uniform storage temperatures in the -120°C to -150°C range using liquid nitrogen. Since then, such dewars have been used at 21st Century Medicine instead of laboratory freezers. Unlike mechanical freezers, ITS dewars have very low power consumption, no heat output, no moving parts, no noise, and limited temperature excursion if they fail.
In 2005 Alcor placed an order with 21st Century Medicine for an ITS dewar large enough to hold 14 neuropatients. After tedious development efforts, the ITS Neurodewar shown schematically in Fig. 11 and photographically in Figs. 12 and 13 was delivered to Alcor in 2008. The unit has been in uneventful operation and evaluation at Alcor since then. The specifications are:
- Storage Volume: 15.5 cubic feet
- Temperature Uniformity (top-to-bottom): 3°C
- Temperature Stability (empty chamber): 2°C during 2″ liquid nitrogen fill
- Operating Temperature Limits: -159°C to -124°C
- Lower Failsafe Temperature: -159°C (0 watts heater power)
- Upper Failsafe Temperature: -124°C (48 watts heater power)
- Maximum Liquid Nitrogen Capacity: 6.0″ or 118 liters
- Liquid Nitrogen Consumption at -159°C: 0.6″ or 12 liters per day
- Liquid Nitrogen Consumption at -145°C: 1.0″ or 20 liters per day
- Liquid Nitrogen Consumption at -140°C: 1.2″ or 24 liters per day
- Rate of Warming Following LN2 Depletion: 1C per hour

Fig. 11. Instead of containers with individual environment control, the ITS Neurodewar system stores 14 smaller uninsulated neurocontainers in a common temperature environment inside a liquid nitrogen dewar. One large storage chamber with thermally-conductive walls ensures a uniform shared storage temperature. Like the neuropod system, small amounts of electrical heat under active control maintain the desired storage temperature in the shared environment.

Fig. 12. (Left) ITS Neurodewar under construction, showing storage chamber with seven storage compartments. (Right) Neurodewar in operation with main lid open, showing closed storage compartment lids.

Fig. 13. (Left) ITS Neurodewar with dual redundant temperature controllers and displays on the right side of the unit. (Right) Neurodewar in operation at Alcor, maintaining an internal temperature of -140°C. The unit automatically refills itself from the connected liquid nitrogen tank.
Comparision of ITS vs. Liquid Nitrogen Immersion Storage
The storage method traditionally used in cryonics is immersion in liquid nitrogen at a temperature of -196°C. Storage vessels that hold liquid nitrogen are kept almost full to the top. ITS systems use dewars that are only partially filled with liquid nitrogen. For example, the ITS dewar of Fig. 13 contains only about 120 liters of liquid nitrogen in a pool at the bottom. This is sufficient to last only 5 days when operating at a temperature of -140°C. In contrast, the tall “Bigfoot” dewars used by Alcor for liquid nitrogen immersion storage contain more than 1000 liters of liquid nitrogen that can last weeks between refills without catastrophic warming. As shown in Fig. 13, ITS dewars can automatically refill themselves from external liquid nitrogen tanks (or be manually refilled if electric power is unavailable), but this is intrinsically less reliable than having the liquid nitrogen already in the dewar.
The ITS Neurodewar of Fig. 13 costs as much as a Bigfoot dewar, but has only one third the neuropatient holding capacity. When operated at -140°C it consumes liquid nitrogen at twice the rate of a Bigfoot dewar. (Liquid nitrogen consumption can be reduced in future units if the operating temperature range is made smaller.) Transfer losses are also expected to be larger due to more frequent filling. Therefore the cost of ITS storage is at least three times that of conventional liquid nitrogen immersion storage.
Whole Body ITS Systems
The same concepts of individual temperature-controlled storage pods, and common temperature storage dewars, can be applied to the design of whole body ITS storage systems. Cryogenic engineer Michael Iarocci and architect Stephen Valentine of the Timeship Project have designed several different whole body ITS systems. Some systems even consume less liquid nitrogen per patient than Bigfoot dewars, but at greater capital cost.
Unresolved Issues
The most important unresolved issue of intermediate temperature storage is how to use it to avoid fracturing. Despite some attempts to avoid fracturing over the last decade, some including months of annealing, acoustic data indicated that fracturing was still occurring during descent to target intermediate storage temperatures. Therefore ITS is presently a means to reduce fracturing, not avoid fracturing. Perhaps ITS is best characterized as a necessary tool to develop future protocols to avoid fracturing. However presently it is not even possible to say whether pod-type storage systems permitting individual temperature control are cost-justified over common temperature environments because it is not known how to use either system to avoid fracturing. There is only a general presumption that a future fracturing avoidance protocol may require lengthy individual temperature conditioning.
A related question is what storage temperature is appropriate for ITS. The lower the temperature the more stable the storage, but the more difficult it is to avoid fracturing. Viscosity at and below the glass transition is so high that chemical reactions can probably be neglected over less than geologic timescales. However a phenomenon called ice nucleation happens at high a rate near the glass transition temperature, and in some studies doesn’t become undetectable until 20 degrees below it. Ice nucleation– the local reorientation of water molecules into nanoscale ice crystals –doesn’t cause immediate structural damage. However it can make avoiding ice growth and associated structural damage during future rewarming more difficult. The extent and significance of ice nucleation in highly concentrated cryoprotectant solutions is still poorly understood (1).
More research is required on fracturing avoidance for large cryopreserved organs and tissues. Valuable research may continue to come from mainstream cryobiology, but some research will need to be specific to cryonics. In the meantime, cryonics organizations face difficult decisions in whether to make an expensive and complex technology that is still unsuccessful in its final objective clinically available. ITS is not unlike cryonics itself.
References
1) B. Wowk, “Thermodynamic aspects of vitrification,” Cryobiology 60 (2010) 11-22.
2) R.C. Merkle, R.A. Freitas, “A Cryopreservation Revival Scenario Using Molecular Nanotechnology,” Cryonics 4th Quarter (2008) 6-8.
3) “Appendix B. A ‘Realistic’ Scenario for Nanotechnological Repair of the Frozen Human Brain,” in Brian Wowk, Michael Darwin, eds., Cryonics: Reaching for Tommorow, Alcor Life Extension Foundation, 1991.
4) G. Fahy, “Vitrification as an approach to cryopreservation: General perspectives,” Cryobiology 51 (2005) 348-414.
5) J.L. Jimenez Rios, Y. Rabin, “Thermal expansion of blood vessels in low cryogenic temperatures, part II: Vitrification with VS55, DP6, and 7.05 M DMSO“, Cryobiology 52 (2006) 284–294.
6) Y. Rabin, P.S. Steif, K.C. Hess, J.L. Jimenez-Rios, M.C. Palastro, “Fracture formation in vitrified thin films of cryoprotectants“, Cryobiology 53 (2006) 75–95.
7) M. Federowicz, H. Hixon, J. Leaf, “Postmortem Examination of Three Cryonic Suspension Patients,” Cryonics September (1984) 16-28.
8) H. Hixon, “Exploring Cracking Phenomena,” Cryonics 1st Quarter (1995) 27-32.
Chronology of Developments Related to Fracturing and Intermediate Temperature Storage
1966
Kroener and Luyet observed fracturing in vitrified glycerol solutions. (C. Kroener, B. Luyet, “Formation of cracks during the vitrification of glycerol solutions and disappearance of the cracks during rewarming,” Biodynamica 10, (1966) 47-52.
1984
Alcor noted fractures in human cryopreservation patients. (Federowicz, M., Hixon, H., and Leaf, J. Postmortem Examination of Three Cryonic Suspension Patients. Cryonics, September, 16-28 (1984))
1990
Fahy published a detailed study of fracturing in large volumes of vitrification solution. (Fahy, G., Saur, J., and Williams, R. Physical Problems with the Vitrification of Large Biological Systems. Cryobiology 27, 492-510 (1990)
1993 March
A detailed discussion and design exercise for a -130ºC “Cold Room” of 100-person capacity took place on the CryoNet email list.
1994
Alcor noted fractures in the brain of a patient following removal from cryopreservation. Various other aspects of the fracturing problem were discussed in the same article, including possible intermediate temperature storage systems, and the development of a new acoustic fracturing monitoring device, the “crackphone.” (Hixon, H. Exploring Cracking Phenomena, Cryonics 1st Qtr. 1995 pags 27-32)
Architect Stephen Valentine began studying Cold Room intermediate temperature storage design concepts as part of a large cryonics facility design that would eventually be called Timeship.
1997
The crackphone acoustic fracturing monitoring device was brought into clinical use by Alcor.
2000
Alcor acquired a -130ºC Harris CryoStar laboratory freezer from GS Laboratory Equipment and began testing its utility for possible storage of neuropatients. (BioTransport Purchases CryoStar Freezer, Cryonics 3rd Qtr. 2000, page 11)
2002
Physicist Brian Wowk and Brookhaven National Laboratory cryogenic engineer Mike Iarocci began an intensive collaboration with architect Stephen Valentine to design intermediate temperature storage systems suitable for cryonics in connection with the Timeship Project.
In summer 2002 an Alcor neuropatient reached the lowest temperature ever recorded without fracturing prior to that time, -128°C. This was attributed to a uniformly low glass transition temperature resulting from excellent cryoprotective perfusion. Professional cryobiologist consultants expressed the opinion that the case may have been the best cryopreservation of any cryonics patient to date, and recommended transfer to the CryoStar freezer for continued slow cooling and annealing for fracture avoidance. In December another patient, A-1034, was also placed into the CryoStar to accommodate wishes of the family for this type of storage.
2003 June
In Ontario, California, presentations were made to the Alcor board of directors by Brian Wowk, Mike Iarocci, and Stephen Valentine on new designs for intermediate temperature storage systems. Alcor purchased and took delivery of an experimental single-patient “neuropod” intermediate temperature storage system developed by Brian Wowk at 21CM (Alcor News #13, July 1st, 2003 and Alcor News #14, August 1st, 2003).
2003 July
The first patient transferred to the CryoStar freezer was transitioned to liquid nitrogen storage because fracture avoidance during slow cooling to -140°C was not successful.
2003 August
Alcor Research Fellow Hugh Hixon began photoelasticity studies of fracturing using a polariscope and polarized light to image stress in cryoprotectant glasses.
Carnegie Mellon University received a $1.3 million grant from the U.S. government to study fracturing during vitrification of tissue for medical applications, resulting in many new and valuable papers in the scientific literature about this subject. (Carnegie Mellon Researchers Developing New Ways to Store Tissue,Organs, Science Daily, August 13, 2003)
2003 October
21st Century Medicine, Inc., constructed a prototype laboratory ITS dewar in which most of the volume of the dewar was converted into a uniform-temperature storage space kept cold by liquid nitrogen.
2004 March
Alcor purchased and took delivery of a “patient rated” neuropod intermediate temperature storage unit for individual neuropatients.
2005 November
Alcor placed an order with 21st Century Medicine, Inc., for a custom ITS dewar large enough to hold 14 neuropatients at a stable intermediate temperature (“ITS Neurodewar”).
2006 January
US Patent 6,988,370, Cryogenic storage system with improved temperature control, was awarded to Mike Iarocci, Stephen Valentine, and Brian Wowk.
An Alcor neuropatient cryopreserved with M22 vitrification solution set a new record for lowest temperature reached without fracturing of -134 degC.
2006 April
Alcor transferred patient A-1034 from the CryoStar freezer to the validated neuropod purchased in 2004.
2007 October
US Patent 7,278,278, Cryogenic storage system, was awarded to Brian Wowk and Mike Iarocci.
2008 December
Alcor took delivery of the ITS neurodewar ordered in 2005. Patient A-1034 was transferred into the new storage unit, and three cryopreserved brains that had been stored by private individuals were accepted into ITS storage.
